Introduction
Malignancies of the nasal cavity is a comparatively rare, accounting for approximately 3% of all head and neck cancers, and exhibit diverse histopathologic features.1–4) squamous cell carcinoma (SCC) is the most common subtype, comprising nearly 50% of nasal cavity cancers, followed by adenocarcinoma, malignant melanoma, adenoid cystic carcinoma and olfactory neuroblastoma.1–4) Despite recent advances in radiotherapeutic techniques, overall survival has shown limited improvement, largely due to local recurrence and complex anatomical constraints.1–3)
Most previously reported cases of nasal cavity SCC involve a single primary tumor or localized recurrence. In contrast, multiple metachronous local occurrences of SCC at different sinonasal and pharyngeal subsites are exceedingly rare, and only a limited number of reports have addressed such atypical clinical courses.5,6) Consequently, the biological behavior, optimal surveillance strategies, and appropriate treatment approaches for patients with repeated local occurrences remain poorly defined.
We report a case of SCC arising metachronously in the oropharynx and nasal cavity, highlighting the need for careful long-term surveillance and individualized treatment strategies in this uncommon disease course.
Case Report
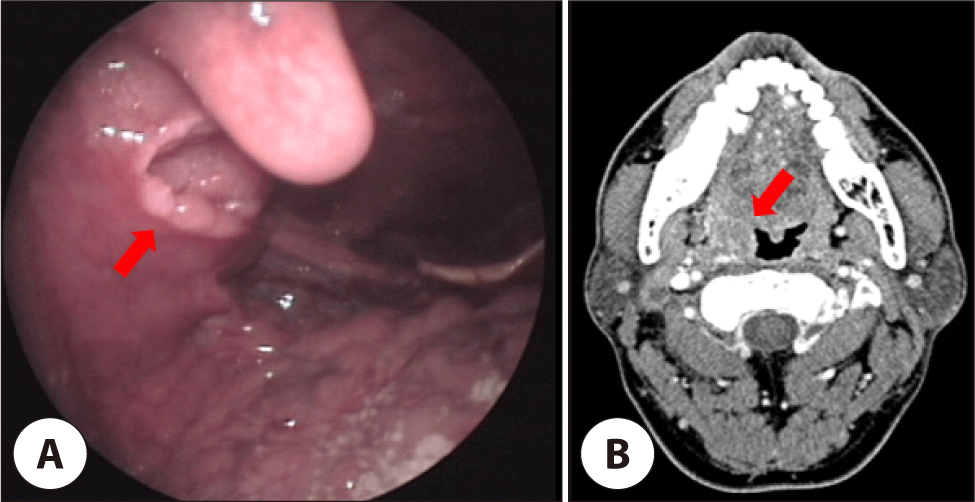
A 55-year-old male with no significant medical or social history was initially diagnosed with HPV-positive SCC of the right palatal tonsil, presenting with throat pain and blood-tinged secretion. Imaging revealed a 1.0×1.9 cm right tonsillar mass with multiple metastatic lymph nodes about 1.0–1.5 cm in the right cervical Level II (Fig. 1). He underwent right radical tonsillectomy and modified radical neck dissection, followed by adjuvant radiotherapy. Pathology revealed predominantly carcinoma in situ with severe dysplasia and equivocal minimal stromal invasion, and no recurrence was observed for 5 years.
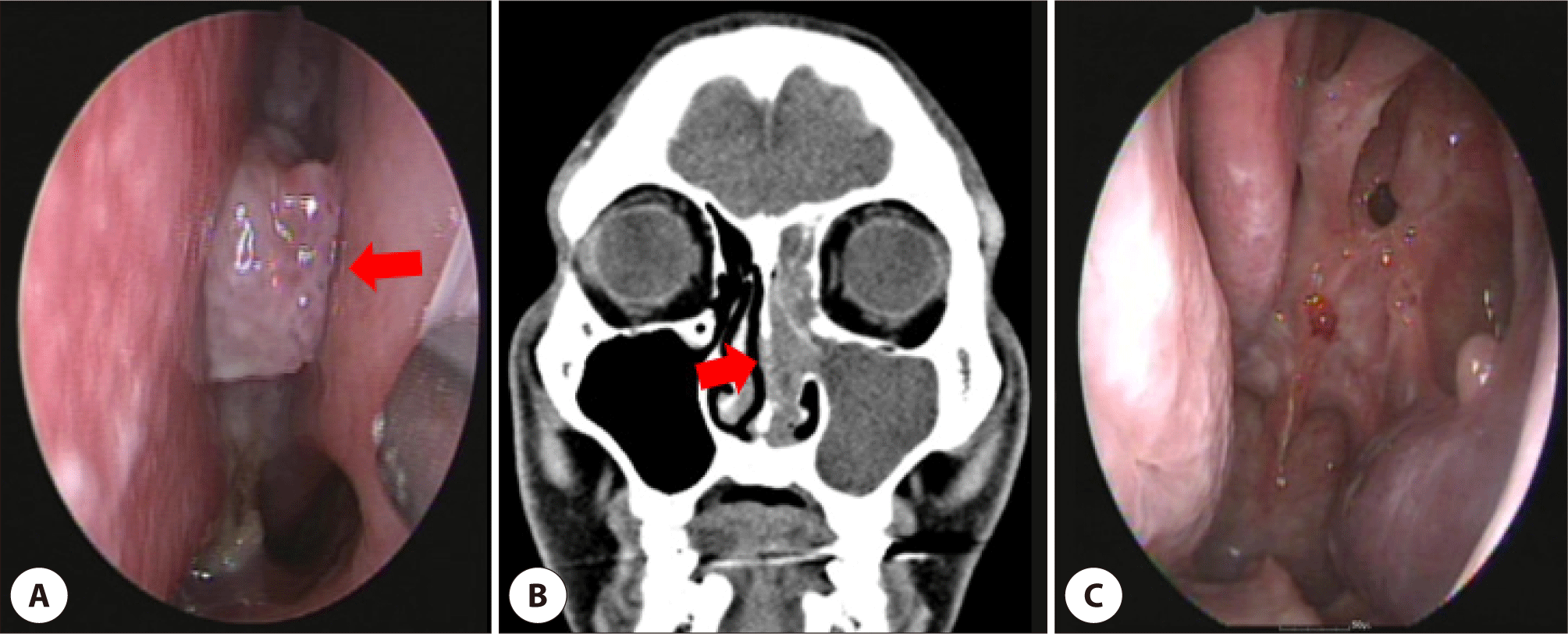
Five years later, he presented progressive bilateral nasal congestion and epistaxis. On nasal endoscopy, a posterior nasal septal mass of about 1.0 cm was observed (Fig. 2A). On computed tomography (CT) of the nasal sinuses, a round mass measuring 1.0×0.6 cm with soft tissue opacification in the nasal septum and left nasal cavity, and haziness of the left frontal sinus was observed (Fig. 2B). On magnetic resonance imaging (MRI), the tumor revealed an irregular margin with surrounding tissues and bone destruction of the posterior nasal septum. Positron emission tomography revealed a hypermetabolic lesion extending to the left ethmoid sinus. The mass was histopathologically diagnosed as SCC punch biopsy. Endoscopic nasal septectomy and left anterior middle turbinectomy were performed, and histopathology confirmed invasive SCC involving the posterior nasal septum and left middle turbinate with negative resection margins (Fig. 2C). No adjuvant radiotherapy was administered, and the patient remained disease-free for 2 years.
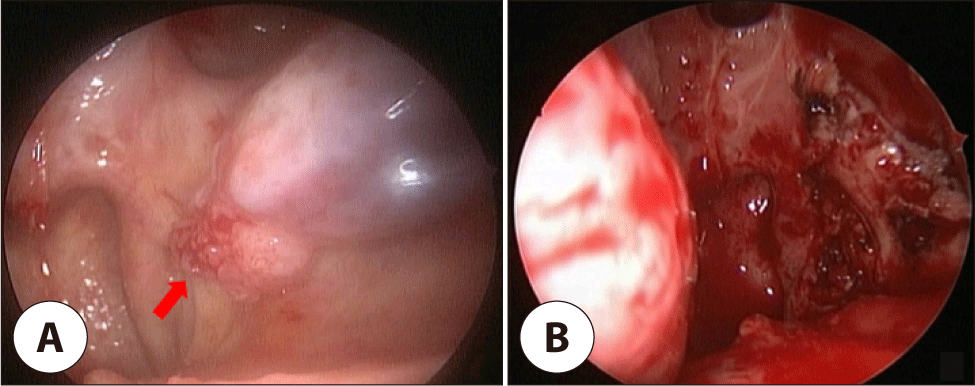
During follow-up, a 0.9 cm bleeding mass of left inferior turbinate was detected and diagnosed as SCC (Fig. 3A). On CT, a soft tissue mass of about 1.0×1.0 cm with irregular margins was detected. MRI revealed a slightly enhanced mass in the left inferior turbinate. Endoscopic left partial inferior turbinectomy was performed (Fig. 3B). Pathology revealed well-differentiated, minimally invasive SCC (invasion depth 0.2 mm) without lymphovascular or perineural invasion, with diffuse p16 positivity. Surgical margins were clear, no adjuvant therapy was given.
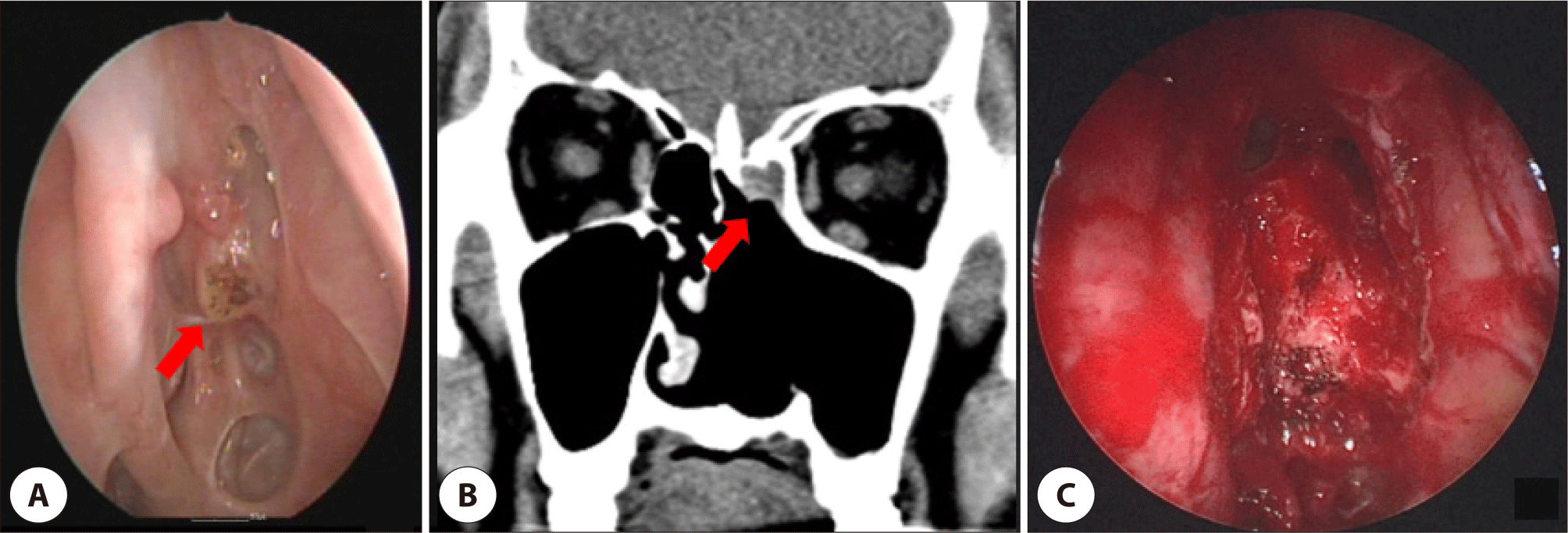
Nine months later, a 1.0 cm lesion in the left frontal recess identified on surveillance nasal endoscopy (Fig. 4A). CT revealed a tumorous soft-tissue lesion accompanied by sinusitis of the left frontal sinus (Fig. 4B). The mass was confirmed to be a SCC. A modified Lothrop operation was performed on both sides of the frontal sinuses. The tumor was removed, and the base of the tumor was drilled to the extent possible (Fig. 4C). Biopsy confirmed well-differentiated SCC with carcinoma in situ components, and immunohistochemistry showed strong p16 positivity with p53 and p63 expression. The patient has remained disease-free for 2 years since the last treatment.
The chronological clinical course, treatments, and follow-up durations are summarized in Table 1.
Discussion
The management of locally recurrent SCC of the nasal cavity has been a challenge due to its low incidence and the lack of consensus regarding optimal treatment strategies.7,8) In the absence of lymphatic and distant metastasis, complete surgical resection with negative margins, with or without adjuvant radiotherapy, is generally considered the mainstay of treatment.8,9)
Unlike malignant mucosal melanoma, which shows frequent local recurrence with skip lesions, sinonasal SCC rarely presents with multiple local occurrences at different sites.1,2,4,9) Therefore, the clinical course of the present case was unusual and resembled the behavior of mucosal melanoma rather than that of typical SCC.
All sinonasal tumors in this case were diagnosed after documented disease-free intervals following treatment of the preceding lesions, supporting a metachronous pattern of tumor development. Although the possibility of occult synchronous lesions at the time of the initial tonsillar cancer diagnosis cannot be entirely excluded, no clinically evident sinonasal abnormalities were identified during the initial diagnostic work-up. The subsequent tumors became clinically apparent only during follow-up or symptom-driven evaluations.
Distinguishing between metachronous multiple primary tumors and local or regional recurrences of SCC is often difficult. The concept of field cancerization suggests that genetically altered mucosa can give rise to multiple tumors over time, either as independent primary tumors or as clonally related lesions through lateral clonal expansion.10,11) Diagnostic tools such as HPV status, p16 immunohistochemistry, TP53 mutation analysis, and comparative genomic profiling have been proposed to aid in this distinction; however, such analyses were not performed in the present case.12–15)
Another important consideration is the possibility of carcinoma arising in association with inverted papilloma, which exhibits distinct histologic and molecular features compared with de novo SCC. In this case, careful histopathologic review revealed no evidence of inverted papilloma in the background mucosa, making an inverted papilloma–associated SCC unlikely.
Previous reports have suggested that irradiation may increase the risk of subsequent SCC development. However, in our case, the nasal cavity was not included in the initial radiotherapy, as the radiation field for the primary tonsillar cancer was confined to the oropharynx and the ipsilateral neck levels II-IV, and there was no history of irradiation at the time of nasal cavity surgery.
Treatment decisions were individualized based on tumor location, extent, prior treatment history, and the feasibility of achieving clear surgical margins. Localized sinonasal lesions in non-irradiated fields were managed with complete endoscopic resection, while adjuvant radiotherapy was reserved for anatomically complex disease involving the frontal recess to improve local control.
In conclusion, the case illustrates a rare clinical course of repeated metachronous SCC involving the pharynx and nasal cavity. Regardless of whether these lesions represent independent primary tumors or clonally related disease, meticulous surgical excision with confirmation of cancer-free margins remains essential. Long-term surveillance is crucial, as sinonasal SCC may occasionally demonstrate atypical patterns of multiple local occurrences despite appropriate initial management.6,7)






