Introduction
Plasmablastic lymphoma (PBL) is a rare disease entity. It is an aggressive form of neoplasm which is characterized with proliferation of large cells similar to immunoblast with a plasmacytic immunophenotype.1) It was initially considered to occur predominantly in the oral cavity of human immunodeficiency virus (HIV)-infected patients, however over the years, it has also been identified in other anatomical sites in HIV-negative individuals.2-4)
Unlike HIV-positive PBL, which shows a marked predilection for the oral cavity involvement, primary location of HIV-negative PBL seemed to be heterogenous. Recently, an increasing number of HIV-negative PBL in several extra-oral sites, including the gastrointestinal tract, soft tissue, bone marrow, skin, lymph nodes, sinuses, lung, and central nervous system have been reported.5)
PBL is characterized by aggressive behavior, recalcitrance to chemotherapy, and poor prognosis. To date, however, little is known about HIV-negative PBL due to its rarity, and there still remains diagnostic and therapeutic challenge. We experienced an unusual case of PBL which occured in nasopharynx of an 85-year-old woman without HIV infection.
Case Report
An 85-year-old female presented increased frequency of epistaxis for two months. The patient also complained of bilateral nasal obstruction, but denied any B symptoms such as fever, night sweats, or weight loss. She underwent heart valve replacement in 2010 and had diabetes.
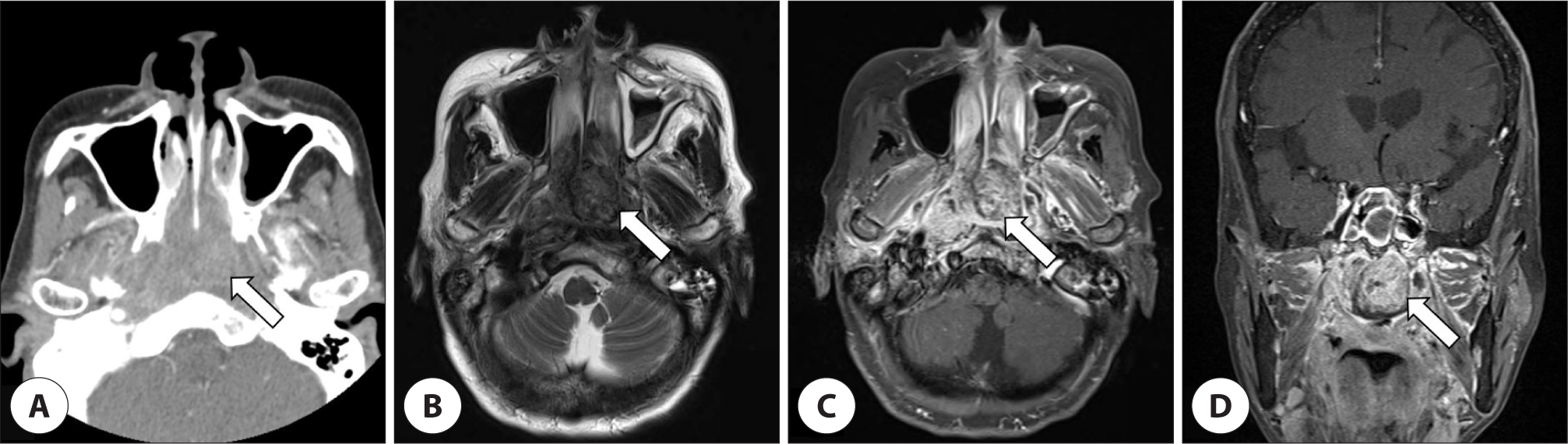
The endoscopic examination revealed a friable, polypoid mass occupying both posterior nasal cavity (Fig. 1). Paranasal sinus computed tomography (CT) and magnetic resonance (MR) images showed that a 3.1×2.3×2.7 cm sized heterogeneous enhancing mass at nasopharynx extended anteriorly into the posterior choanae without bone destruction (Fig. 2).
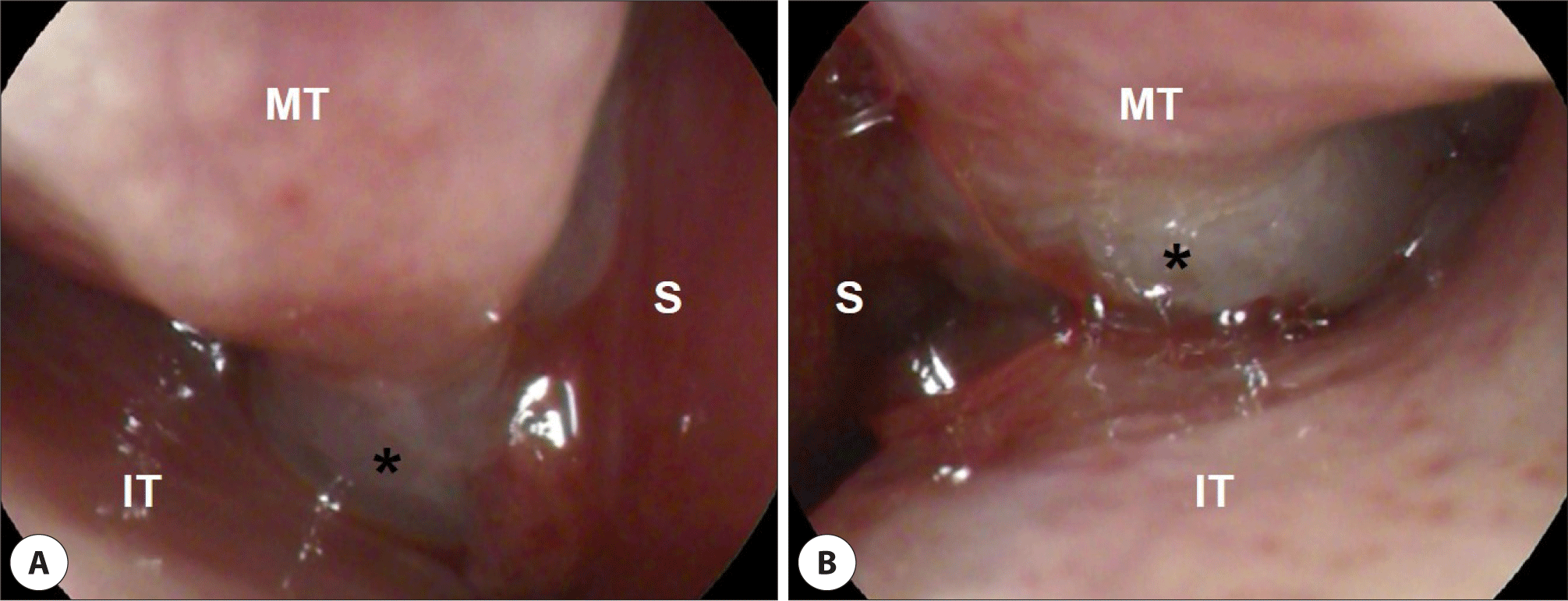
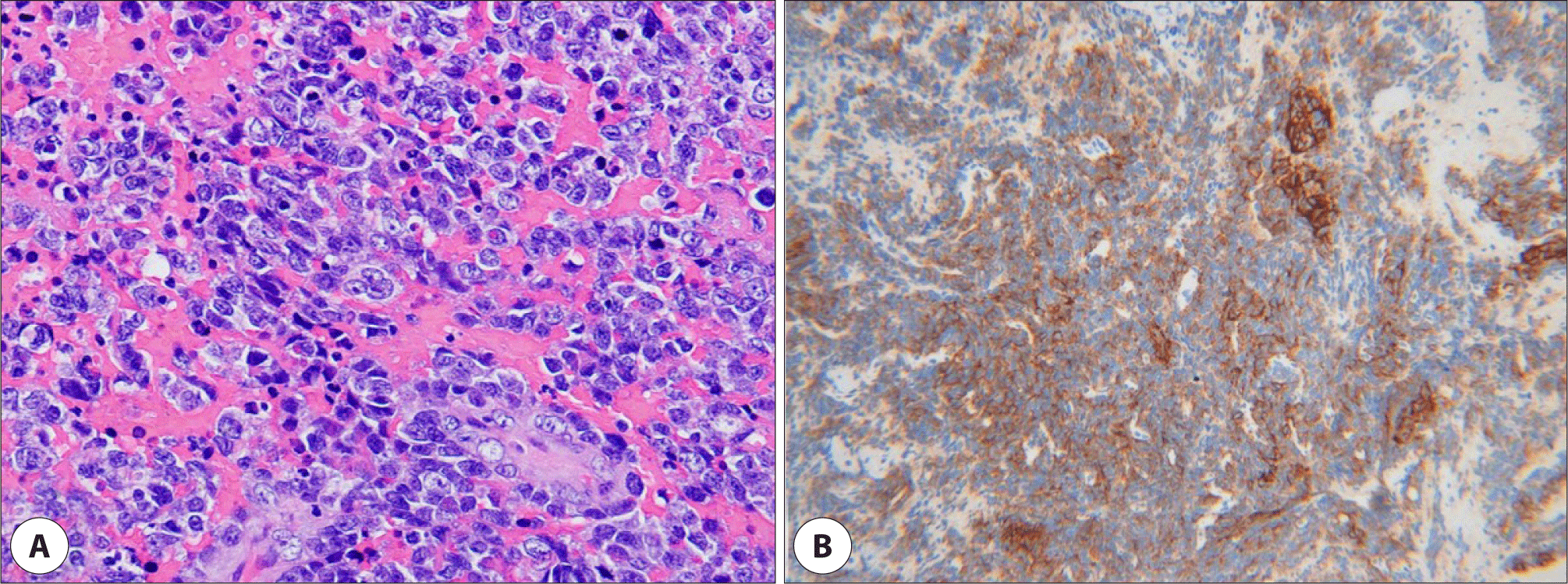
A transnasal endoscopic biopsy was performed on an outpatient basis, but the results suggested no evidence of malignancy. The patient was taken to the operating room where wide resection of the tumor was performed and the remnant was debrided. In histopathologic examination, the tumor cells were composed of monotonous proliferation of large lymphoid cells with immunoblastic features. Immunochemical staining showed that the tumor cells were reactive toward CD138 and leukocyte common antigen (LCA), and negative toward CD56, anaplastic lymphoma kinase (ALK), cytokeratin (CK), and vimentin (Fig. 3). In situ hybridization tests including Epstein-Barr virus encoded RNA (EBER), kappa and lamda chain showed negative, and Ki-67 proliferative index was focally high. These morphologic and immunochemical findings were consistent with PBL. The subsequent positron emission tomography-scan showed hypermetabolic uptake in the nasopharynx, left nasal cavity, and right neck level II. A bone marrow biopsy from both posterior iliac crests revealed 5%–20% hypocellular marrow with foci of lymphoid cells (CD138, LCA positive) among normal hematopoietic cells.
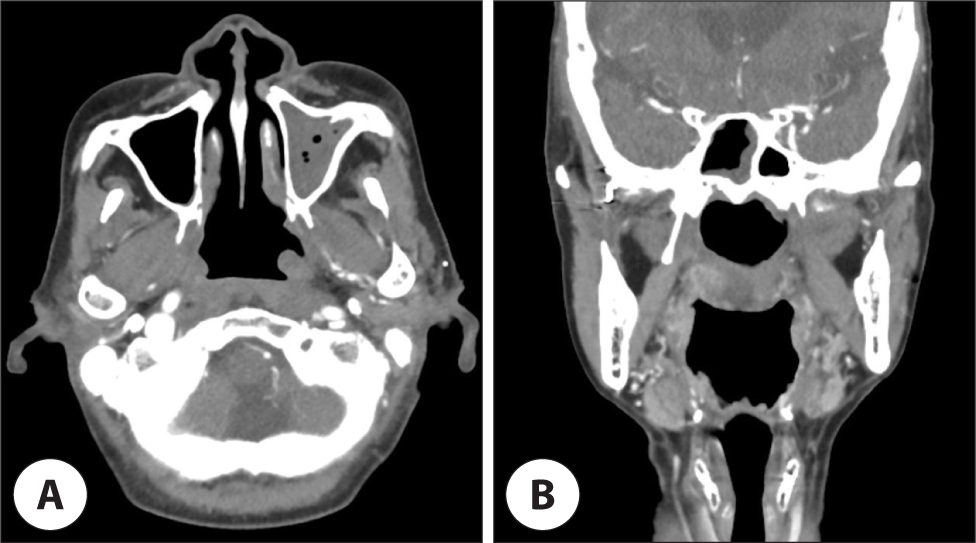
According to the Ann Arbor staging system, the patient was diagnosed as stage IV, and underwent with six cycles of etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (da-EPOCH) chemotherapy. The tumor showed partial response to treatment for a while (Fig. 4). However, the disease progression was observed soon after. Additional concurrent chemoradiation therapy with cisplatin was performed, but the patient’s general condition became poor and developed sepsis after urinary tract infection. The patient expired 9 months after the initial diagnosis.
Discussion
In 1997, Delecluse et al. first described a distinct subtype of diffuse large B cell lymphoma (DLBCL) occuring in the oral cavity of HIV-infected patients and termed them as PBL in reference to their blastoid morphology and plasmacytic immunophenotype.2) This lymphoma was previously classified as a variant of DLBCL, and it is currently thought to be the member of a separate diagnostic entity which is lymphoid neoplasm with plasmablastic differentiation.1)
PBL was originally described to occur predominantly in HIV-infected patients.2) Over time, it has also been identified in patients with other causes of immunodeficiency such as patients with solid organ transplant or elderly with age-associated immunosenescence or patients receiving immunosuppressive therapy. Furthermore, PBL is also known to be associated with immunocompetent individuals.3,4) In recent studies, about 5% of the PBL cases seemed to be immunocompetent whose immune system has no apparent defect.4)
The clinical manifestations of PBL patients vary with different immunological status. Several studies comparing the clinicopathological features of PBL patients with and without HIV infection reported that the HIV-associated PBL group generally showed early onset, male predominance, predilection for oral involvement and response to chemotherapy, whereas the HIV-negative PBL group showed elderly onset, high rates of extra-nodal involvement and short overall survival.5,6) The most commonly affected site in HIV-associated PBL is oral cavity, followed by gastrointestinal tract, lymph nodes, and skin. Although a similar feature is observed in HIV-negative PBL, it appears to be more heterogeneous than in HIV-associated PBL. The majority (89%) of HIV-negative PBL patients reported at least one extranodal site of involvement, and not seemed to be as highly associated with oral involvement (21%) as in HIV-positive patients (58%). In both groups, most patients presented with disease in clinically advanced stage of Ann Arbor stage III or IV with frequent involvement of bone marrow at diagnosis.5,7,8)
Given its rarity and lack of expression of the markers that are usually used for lymphoma, diagnosis of PBL still remains challenging for both clinicians and pathologists. PBL is thought to arise from an activated and terminally differentiated post-germinal center B-cells that has acquired almost fully mature plasma cell phenotype. Hence, markers of plasmacytic differentiation including CD38, CD138, IRF4/MUM1, PRDM1/BLIMP1, and XBP1 are typically expressed, whereas leukocyte common antigen (CD45) and the B-cell markers including CD20, CD79a, and PAX5 are usually absent or only weakly positive. Unlike in true plasma cell tumors, CD56 is usually not expressed in PBL. The Ki-67 proliferation index is usually high (>90%), and in situ hybridization for EBER shows positivity in around 80% of the HIV-positive patients and around 50% of the HIV-negative patients.7,9,10)
Although there is a wide range of disease for the differential diagnosis, the main consideration is plasmablastic myeloma (PBM). Since morphologic and immunophenotypic features of PBM and PBL are almost identical, the distinction between these two entities usually depend on clinical, laboratory, and radiologic correlations. The presence of paraproteinemia, hypercalcemia, renal dysfunction, anemia, and osteolytic lesions favors the diagnosis of PBM over PBL. Meanwhile, positivity for EBV, HIV infection and a high Ki-67 proliferation index are more frequently associated with PBL. Cyclin D1 is expressed in about one third to half of PBM cases but generally negative in PBL. In addition, there are other important entities to differentiate from PBL, such as primary effusion lymphoma, ALK- positive DLBCL, and DLBCL with plasmacytoid differentiation, which make the diagnosis more challenging.7,10,11)
Up to date, standard regimen for PBL treatment has not been established. Due to early dissemination, recalcitrance to chemotherapy, and high rates of relapse, PBL is known to have poor prognosis under currently available treatments with median overall survival of around 8 months.8,11) In contrast to treatment for DLBCL, conventional chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP-like regimen is considered inadequate to obtain sufficient therapeutic response. Thus, the National Comprehensive Cancer Network (NCCN) group recommends more intensive regimens, such as infusional EPOCH, cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate/ifosfamide, etoposide, and high-dose cytarabine, or hyper-hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone, and high-dose methotrexate and cytarabine. Nervertheless, some studies have reported the survival rate between patients treated with more intensive regimens and those treated with CHOP or CHOP-like regimens showed no significant difference.8,12) Recently, new agents such as bortezomib which is widely used for the treatment of multiple myeloma, and induction chemotherapy followed by autologous stem cell transplantation have been suggested as alternative options, but it is thought that further research is needed.7)
Conclusion
As a rare and aggressive subtype of non-Hodgkin’s lymphoma, PBL has a feature of proliferation of large cells which look like immunoblast and the expression of a plasma cell immunophenotype. Despite its common association with HIV infection, PBL in HIV-negative individuals is reported to be increasing. In this case, the authors diagnosed the tumor arising from the nasopharynx of HIV-negative patient as PBL based on the its immunoblastic morphology, positivity for CD138 and focal high ki67 index, negativity for CD56, ALK, CK, and vimentin, even though EBER is not detected. Because of the rarity and distinct profile of this entity, clinicians and pathologists should have a high index of suspicion for its identification.






